
|
   
   
|


|
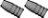
Ok, so i did a burn test with a small propane torch and it looks like there is a good bit of antimony in my alloy as it splashes a white/green flame off of the top
Never heard of a "burn test" on lead? White to me would be Phosphorous or magnesium and green would be copper. I would think it would melt so fast you would not get any kind of read. And remember that lead over 1100-1200 degrees is puts out toxic fumes.
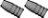
Size and appearance wise, I’d say it’s a radioisotope container. All the ones I’ve gotten and analyzed that weren’t pure lead (and those were uncommon) were around 3% Sb with little or no tin.
If you cast from the containers only, I’d guess you got high alloy temp frosting from the antimony (my own 3% sb alloy will do that) and, at lower pot temp, poor fill out from the lack of tin. The hardness would be about Brinell 10 or 11.
If you used the harder ingots, I’d guess that alloy to be higher Sb and even more prone to frosting, and again low tin content. I’ll qualify that with the observation that hardness testing was described to me by a maker of hardness testers as being inconsistent due to the variable cooling rate dependent on the mass and shape of the ingot, compared to that of bullets, so that the alloy could be the same.
1-2% Sn added to either should cast with good fill out at lower pot temps if you don’t want the frosting.
Last edited by kevin c; 05-07-2024 at 06:36 AM. Reason: Clarification added
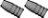
As Kevin mentions, the cooling rate does affect the hardness of an ingot. I was given a good number of 5 pound ingots with the comment that the person was not sure what the alloy was in the ingots. I used the Cabin Tree tester and found I had three general hardness test results. I melted a sample from the three different groups, sent it off to BNE and found that they were all the same alloy.
That is why, sample prep is very important when using the hardness test units. Perhaps that is why some suggest taking more than one test on the same ingot to average the readings. The large and small ingots have a different cooling rate. That is sufficient reason to use one bullet mold to cast sample bullets for testing.
I don't find testing hardness of ingots is a reliable method to predict the hardness of bullets cast from those ingots. Close but not dead on. Maybe close enough. I test bullets for hardness not ingots. When I am going to cast ingots that will be used straight, I cast a few bullets at the same time and test the bullets and mark the ingots with the hardness of the bullets.
Tim
Words are weapons sharper than knives - INXS
The pen is mightier than the sword - Edward Bulwer-Lytton
The tongue is mightier than the blade - Euripides
Kevin, thank you so much that is spot on with what im seeing the only way i can get them to cast decent at lower temps is to flux and scoop off the top layer …
Do you have a picture of the dross? Is it thick and gravely even after fluxing at 700 degrees? It could be calcium contamination.
Years ago I was melting some isotope containers and everything was going smoothly till I added one and got a thick dross on top that did not want to flux back in so like you I scooped it off. Looking back I am now thinking one container was calcium contaminated which mixes with antimony and forms an insoluble intermettalic compound. This can be skimmed off but the dross is toxic and gives off poisonous gasses when exposed to moisture. Do any smelting outside and as soon as the dross is cool put it into an airtight container. Not sure how to dispose of it.
Are all of the "ingots" solid or is that a pic of a hollow cylindrical isotope containers?
You might look here for your containers:
http://www.fellingfamily.net/isolead/
Medium cylinder from your outside dimensions. Maybe check the weight to refine your search.
Last edited by jsizemore; 05-08-2024 at 06:33 PM.
The dross was the calcium / antimony intermetallic. IIRC correctly once it was removed the lead behaved normally when remelted but it will be a little softer since you also removed some antimony. Melting radioisotope vials can be like playing roulette. I had a nice pot of lead behaving normally then when I added one vial I got all of the crud on top. Not sure how to tell the bad containers from the good ones.
I didn't know the dross could give off poisonous gasses when this happened to me but I smelt outside and didn't notice anything.
Unless you actually had the dross scanned, I would be hesitant to say it was contaminated with calcium. The site referenced to help ID isotope generators did not mention calcium being used. While you might be correct, an XRF scan would be the only certain way to confirm your opinion.
Keep in mind that an alloy rich in antimony may not go fully into solution at only 700 degrees. Still, being safe in handling the dross is very important.
I am just speculating on the calcium but whatever happened gave a thick dross when the one "contaminated" vial was added and fluxing didn't help. I believe if an alloy just has calcium it casts OK and this is what is used in batteries. Calcium is only a problem when it mixes with the antimony in just about all of our alloys.
When this happened to me I was a casting newbie and didn't understand what happened. Later I bought the Lyman cast bullet handbook where what happened was explained and had a warning of the hazards. They even had a picture of a calcium contaminated pot showing the distinctive dross.
I do not doubt your observations, just have to wonder why when the major material is either pure lead, or perhaps lead-tin-antimony one would suddenly find a container made with a calcium alloy. I can understand a problem with the epoxy paint on the exterior, and the construction adhesive used to keep the plastic liner in place being a problem. I do understand the various sources listed in Lyman CBH 3rd ed, but these containers have been around for a very long time and would have likely been mentioned as a possible source if it was true. Perhaps someone else has had a similar issue, but so far, none have come forward.
| BP | Bronze Point | IMR | Improved Military Rifle | PTD | Pointed |
| BR | Bench Rest | M | Magnum | RN | Round Nose |
| BT | Boat Tail | PL | Power-Lokt | SP | Soft Point |
| C | Compressed Charge | PR | Primer | SPCL | Soft Point "Core-Lokt" |
| HP | Hollow Point | PSPCL | Pointed Soft Point "Core Lokt" | C.O.L. | Cartridge Overall Length |
| PSP | Pointed Soft Point | Spz | Spitzer Point | SBT | Spitzer Boat Tail |
| LRN | Lead Round Nose | LWC | Lead Wad Cutter | LSWC | Lead Semi Wad Cutter |
| GC | Gas Check |